Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – USA News Group News Commentary – As World Cancer Day (Feb. 4) fades into the rearview, attention is shifting to the latest discussions among oncology experts worldwide. A recent report from The New York Times raised pressing questions about cancer and its treatments, including the role of pollution—such as microplastics—in rising cancer rates, genetic mutations, and inflammation. Over in the UK, The Guardian highlighted the growing concern over lung cancer cases among non-smokers, calling for deeper investigations into the link between air pollution and the disease. Meanwhile, experts are sounding alarms over the rapid increase in colorectal cancer diagnoses among younger populations. Another report pointed to the EU lagging behind the USA in oncology innovation. Amid these developments, biotech firms remain at the forefront of cancer research, with recent advancements coming from Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Absci Corporation (NASDAQ: ABSI), GSK plc (NYSE: GSK), GeoVax Labs, Inc. (NASDAQ: GOVX), and Zentalis Pharmaceuticals, Inc. (NASDAQ: ZNTL).
The article continued: The Center for Innovation and Translation of Point of Care Technologies for Equitable Cancer Care (CITEC) has launched a global initiative to drive progress in cancer detection technologies. According to DelveInsight Business Research, the global cancer therapy market is on track for substantial growth, with a projected compound annual growth rate (CAGR) of 9.12%, bringing its estimated value to $285.96 billion by 2030.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), a clinical-stage company focused on immunotherapy for cancer, recently presented new data from its GOBLET study at the ASCO GI symposium. The company showcased pelareorep, an innovative immunotherapy designed to train the immune system to attack cancer by converting treatment-resistant “cold” tumors into “hot” tumors that respond more effectively to therapy.
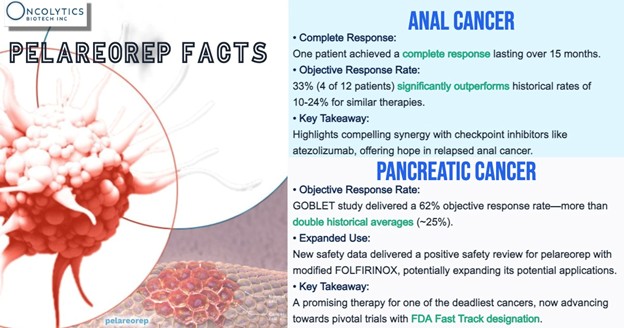
These latest results build on Oncolytics’ progress in 2025, following a key regulatory approval that paves the way for advancing its pancreatic cancer treatment. The company is moving forward with the next phase of its study of pelareorep in combination with mFOLFIRINOX, with or without atezolizumab, for newly diagnosed pancreatic adenocarcinoma (PDAC) patients. A positive safety review has brought Oncolytics closer to completing full enrollment for Cohort 5 of the GOBLET trial.
At ASCO GI, Oncolytics also shared encouraging data on relapsed anal cancer. Of 12 evaluable patients, four experienced a partial response, translating to a 33% response rate, while one patient achieved a complete response with no detectable cancer for over 15 months. By comparison, standard treatments typically show response rates between 10-24%, highlighting pelareorep’s potential to improve outcomes in some of the most challenging cancer cases.
“In relapsed anal cancer, the efficacy signal that was initially reported continues to outperform historical control trials with the inclusion of additional patients,” said Thomas Heineman, M.D., Ph.D., Chief Medical Officer for Oncolytics Biotech. “Importantly, the complete response we observed previously continued beyond the 12 months initially reported. Together, these results point to a clinically meaningful synergy between pelareorep and checkpoint inhibitors like atezolizumab.”
Pelareorep has shown promising potential in enhancing treatment outcomes for patients battling pancreatic cancer, a particularly aggressive disease. At the ASCO GI presentation, Oncolytics highlighted fresh safety data, demonstrating that pelareorep can be effectively combined with modified FOLFIRINOX, a commonly used chemotherapy regimen for pancreatic cancer patients.
“Our new safety data indicate its ability to also be combined with modified FOLFIRINOX, thus expanding its potential to benefit patients with metastatic pancreatic cancer,” added Dr. Heineman. “We will continue to provide updates on the safety and efficacy of pelareorep-based combination therapy from these cohorts as they become available.”
These findings build on previous results from the GOBLET study, where the combination of pelareorep with atezolizumab, gemcitabine, and nab-paclitaxel demonstrated an objective response rate of 62%—more than twice the historical average of 25%. This promising data played a key role in pelareorep receiving FDA Fast Track designation in 2022, underscoring its potential to address the urgent need for more effective pancreatic cancer treatments.
The ability to combine pelareorep with modified FOLFIRINOX marks a significant advancement in expanding available treatment options for metastatic pancreatic cancer. These results not only reinforce the drug’s versatility but also suggest its potential to improve outcomes when paired with various standard therapies.
As Oncolytics Biotech progresses its clinical programs toward potential pivotal trials, the accumulating evidence continues to position pelareorep as a groundbreaking therapeutic candidate for patients facing limited treatment options.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Absci Corporation (NASDAQ: ABSI) and Owkin have joined forces to accelerate drug discovery using AI. Their partnership combines Absci’s generative AI platform, which designs new antibody therapies, with Owkin’s predictive AI models, which help select and validate drug targets using biomedical data. Together, they aim to develop new treatments in immuno-oncology, immunology, and inflammation, streamlining the process from research to clinical development. This collaboration adds to Absci’s growing list of partnerships with major pharma companies like AstraZeneca and Merck, while Owkin continues to work with industry leaders such as Sanofi and BMS to advance AI-driven drug discovery.
“At Absci, we’re constantly pushing the boundaries of innovation to bring better biologics to patients faster,” said Sean McClain, Founder and CEO of Absci. “By combining Absci’s AI de novo design expertise with Owkin’s world-class predictive AI target discovery technology, we have a unique opportunity to design first-in-class and potentially transformative therapeutics for patients in need.”
GSK plc (NYSE: GSK) announced its acquisition of IDRx, a Boston-based biotech company focused on precision treatments for gastrointestinal stromal tumors (GIST), for $1 billion upfront, with a potential $150 million milestone payment. The deal includes IDRX-42, a promising drug designed to target both primary and secondary KIT mutations, which drive tumor growth in about 80% of GIST cases. Currently, no approved tyrosine kinase inhibitor (TKI) treatments effectively target all these mutations, but IDRX-42 has shown strong potential in clinical trials. In an ongoing Phase I/Ib study, it demonstrated a 29% response rate in advanced GIST patients, rising to 53% in those who had only one prior treatment, with manageable side effects. GSK sees this acquisition as a key step in expanding its oncology portfolio, which already includes trials for GI cancer treatments like dostarlimab and GSK’227. The move aligns with GSK’s long-term strategy to develop cutting-edge therapies and drive growth beyond 2031.
“IDRX-42 complements our growing portfolio in gastrointestinal cancers,” said Luke Miels, Chief Commercial Officer of GSK. “This acquisition is consistent with our approach of acquiring assets that address validated targets and where there is clear unmet medical need, despite existing approved products.”
GeoVax Labs, Inc. (NASDAQ: GOVX) is integrating artificial intelligence (AI) into its cancer immunotherapy and vaccine programs to accelerate research, improve treatments, and enhance efficiency. By leveraging AI, GeoVax is refining its cancer therapy platform, optimizing treatment strategies for solid tumors, and improving patient targeting for better outcomes. The company’s AI-driven approach also streamlines manufacturing and supply chains, ensuring faster and more effective delivery of its therapies. As part of its commitment to innovation, GeoVax aligns with President Trump’s Stargate Initiative, which emphasizes AI’s role in advancing U.S. healthcare and biosecurity. This AI-driven strategy positions GeoVax at the forefront of oncology innovation, potentially reducing development timelines and increasing the impact of its cancer treatments.
“President Trump’s Stargate Initiative exemplifies leadership in harnessing AI to address critical healthcare challenges,” said David Dodd, Chairman and CEO of GeoVax. “GeoVax is honored to support this initiative, highlighting its application of AI to develop vaccines and therapies that save lives and enhance biosecurity. We are proud to contribute to a future where the United States leads the world in healthcare innovation.”
Zentalis Pharmaceuticals, Inc. (NASDAQ: ZNTL) is making significant progress in its oncology research, particularly in developing azenosertib, a promising drug for treating platinum-resistant ovarian cancer (PROC). Recent clinical trials, including ZN-c3-001, MAMMOTH, and DENALI, have shown encouraging results, with azenosertib demonstrating strong response rates and manageable side effects. The company has aligned with the FDA on its DENALI Part 2 study, set to begin in early 2025, which could support accelerated approval if successful. Notably, in PROC patients with a specific biomarker (Cyclin E1+), azenosertib achieved response rates of over 30%, with patients seeing improvements in disease control. While the company is discontinuing some combination studies due to strategic priorities, the overall findings reinforce azenosertib’s potential as a new targeted therapy for hard-to-treat cancers.
“We are excited to outline a clear path for Zentalis to bring azenosertib to patients with Cyclin E1+ PROC,” said Ingmar Bruns, M.D., Chief Medical Officer of Zentalis. “In a patient population with a clear unmet medical need, the monotherapy data showed a meaningful and consistent improvement in responses as compared to historical data from current monotherapy chemo standard of care, across multiple studies, in heavily-pretreated patients at the 400mg QD 5:2 intermittent dose.”
CONTACT:
USA NEWS GROUP
(604) 265-2873
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice commencing immediately and ongoing. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material, including this article, which is disseminated by MIQ has been approved by Oncolytics Biotech Inc.; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
creator solana token

_-_IMG_1104.jpg?w=1200&resize=1200,0&ssl=1)




